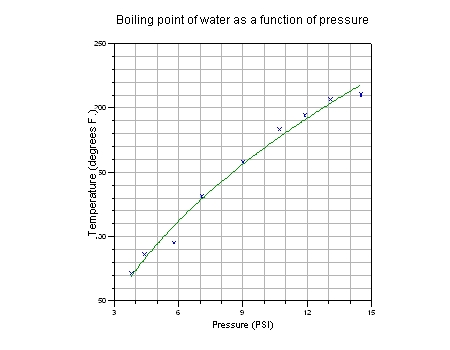
This example models the relationship between the boiling
point of water and the ambient pressure.
As ambient pressure decreases, the boiling point of water
also decreases. This relationship can be modeled by
Clapeyron's Equation which shows that the boiling temperature
(in degrees Fahrenheit) is related to pressure as follows:
Temperature = b / log(Pressure/a) - 459.7
where a and b are parameters whose values are
to be determined by the analysis.
Here are the NLREG statements to fit this function:
Title "Boiling point of water as a function of pressure";
Variable Pressure; // Pressure in vessel (PSI)
Variable Temp; // Temperature in degrees Fahrenheit
Parameter a = 1700; // Specify starting values
Parameter b = -3200;
Function Temp = b / log(Pressure/a) - 459.7;
Plot xLabel="Pressure (PSI)",yLabel="Temperature (degrees F.)";
Data;
[ data goes here ]
Here is a plot generated by NLREG showing the function fitted to a
set of pressure and temperature measurements: